
All newly made proteins must fold into their three-dimensional structures to function, and many must reach destinations other than the cytosol (where protein synthesis occurs). In addition, proteins often undergo post-translational modification in response to cellular signals.
Errors in these processes cause diseases ranging from Alzheimer's to diabetes. Therefore, understanding the mechanism and regulation of protein folding, protein translocation, and protein processing is an integral part of modern molecular and cell biology.
Research topics of faculty in this area include the unfolded protein response, the human blood clotting system, the structure and function of molecular chaperones, the heat shock response, protein misfolding in aging and disease, protein transport in the secretory pathway, protein targeting, organelle biogenesis, and protein design and engineering.
Ryan Baldridge, PhD
Mechanisms of membrane-bound protein quality control systems
Lydia Freddolino, PhD
Structure prediction for macromolecular complexes
Phyllis Hanson, MD, PhD
Protein-protein and protein-membrane interactions involved in membrane trafficking and organelle structure
James Morrissey, PhD
Biochemistry of the human blood clotting system; structural studies of protein-membrane complexes
Stephen Ragsdale, PhD
Interactions and processing of proteins involved in heme metabolism, the circadian clock, the global carbon cycle, and methylmercury
Zhaohui Xu, PhD
Structural biology and molecular mechanisms of protein folding and trafficking in eukaryotic cells
James Bardwell, PhD
Roles of molecular chaperones and disulfide catalysts in protein folding; experimental evolution of protein folding
Ursula Jakob, PhD
Biochemical aspects of the bacterial response to oxidative stress
Matthew Chapman, PhD
Biogenesis of bacterial amyloid fibers called curli
Peter Todd, MD, PhD
Roles of RNA and RNA processing in neurological disease
Anthony Vecchiarelli, PhD
Molecular mechanisms underlying the subcellular organization of bacterial organelles
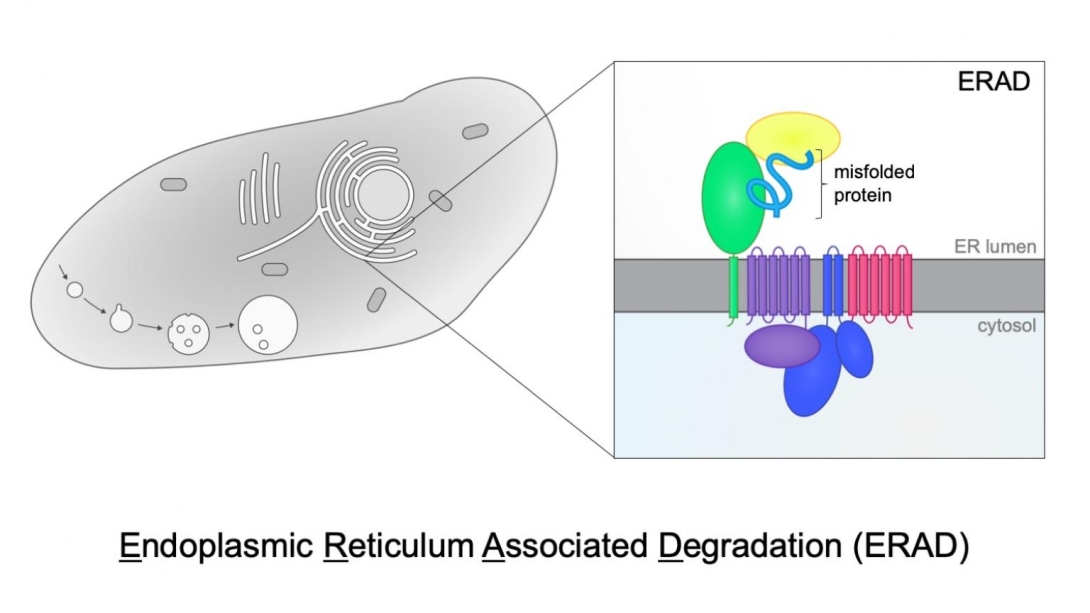
The Baldridge lab studies ERAD (Endoplasmic Reticulum Associated Degradation) of misfolded proteins.
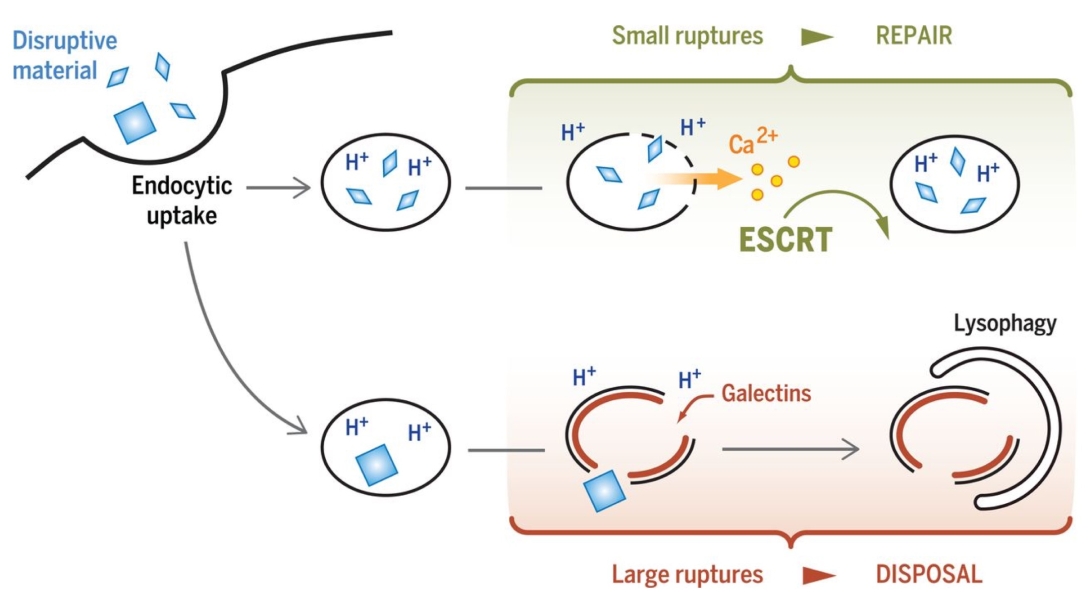
The role of the ESCRT (Endosomal Sorting Complexes Required for Transport) machinery in membrane repair is under investigation in the Hanson lab.
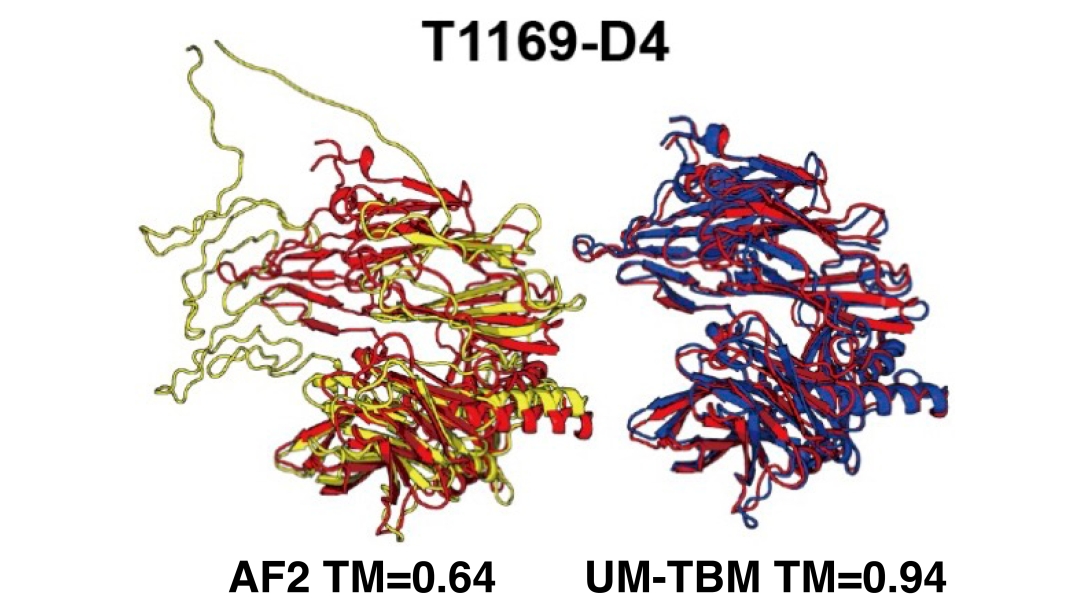
The Freddolino lab is at the forefront of macromolecular structure prediction, as shown in this example of a CASP15 target where their prediction (UM-TBM) performed substantially better than AlphaFold2 (AF2).